
Steel corrosion can be costly, dangerous, and unsightly. It’s important to make sure the steel used in your project is utilizing an effective method for protection against corrosion, abrasion, and weather conditions. Hot dip galvanizing is the process of factory dipping of fabricated steel into a kettle of molten zinc with the goal of preventing this corrosion, and creating a steel product that stays structurally sound and aesthetically pleasing for years to come. Hot dip galvanizing is often the preferred method of corrosion protection for steel for a number of its unique benefits, including:
Durability & Corrosion Protection
First, hot dip galvanizing is a great investment because of its durability and protection from even the harshest environments. This protective zinc alloy becomes tightly bonded to the steel, creating a layered protection with a bond strength of around 3,600 psi. In addition to the corrosion protection in any environment, this alloy also provides excellent abrasion resistance that is harder than the steel, protecting it from any damage that might occur during the shipment or construction process.
Additionally, hot dip galvanizing is the ONLY system that protects the steel on the inside and the outside. Ensuring that the inside of fabricated steel is galvanized can be crucial to the integrity and life of the steel. Also, because the steel is completely submerged in our zinc bath, there is a uniform protection on the entirety of the fabricated steel, while other methods such as liquid coatings often cause the protective coating along the edges to be thinner. Utilizing hot dip galvanizing is the only process that ensures this complete and uniform protection for steel.
Cost
Hot dip galvanizing also has several cost benefits. The overall material and application generally costs less than other corrosion protection methods. It also can be completed in less time due to the factory immersion process, saving construction time costs as well.
It also has lifetime cost benefits, as hot dip galvanized steel requires much less maintenance over time. In this chart, you can see the impressive amount of time hot dip galvanizing protects the steel from corrosion before first maintenance is required.
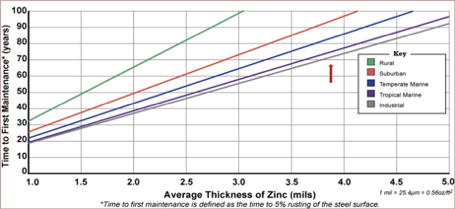
Sustainability
Both zinc and steel are 100% recyclable, making hot dip galvanizing a very ‘green’ process. Zinc is natural, abundant, and essential to life. Therefore, the use of steel is not introducing any harmful substances into the environment. Zinc and steel can also be recycled to be used many times over in different projects, so even at the end of a building or structure’s life, that material can be used again. In fact, 30% of the world’s zinc supply is recycled. This way, you can ensure that the materials used will not create waste. Hot dip galvanizing also uses less natural resources and creates less harmful emissions into the atmosphere, contributing to the overall environmental friendliness of your construction job.
Availability & Versatility
Hot dip galvanized steel is both readily available and accessible, as well as versatile for a variety of projects. Steel of many different shapes and sizes can be galvanized. Because of the total immersion in the zinc bath, even intricate shapes can be fully galvanized and protected from corrosion. This makes hot dip galvanizing a great choice for steel components that have a unique or complicated shape.
Additionally, because galvanizing occurs factory in a controlled environment, the process can be completed in any weather conditions, while other processes can’t. This allows construction projects to move quicker when the galvanizing can be completed any day, any time of year. The average turnaround time for hot dip galvanized steel ready for use is about 3-5 days, quicker than many other processes.
Contact Duncan Galvanizing
Duncan Galvanizing is the Northeast’s trusted provider of hot dip galvanizing, color galvanizing, and more. Contact Howie Levine for help with specifications or Seth Dubinsky to request a quote at (617) 389-8440 or fill out an online form to get started.



 The size of zinc baths varies from galvanizer to galvanizer and this restricts the size of steel that can be galvanized. When choosing a galvanizer, make sure that the size of the zinc bath is big enough to accommodate the size of your products such as large structural shapes.
The size of zinc baths varies from galvanizer to galvanizer and this restricts the size of steel that can be galvanized. When choosing a galvanizer, make sure that the size of the zinc bath is big enough to accommodate the size of your products such as large structural shapes.