Hot-dip galvanizing and metallizing are both great and effective ways to protect steel against corrosion. However, they do have some significant differences, and it’s important to know all the details before choosing a method for your project’s corrosion protection. We broke down the differences and the main pros and cons of each so you can make an educated choice.
Galvanizing vs. Metallizing
When metal is hot-dip galvanized, it is brought to a galvanizing plant. Here, the metal is cleaned, prepped, and then it is completely submerged into a kettle of molten zinc to form a uniform layer of corrosion protection by alloying with the steel. Thermal spray zinc, also known as metallizing, is the process of coating steel by transporting molten zinc by a gas stream to a properly prepared substrate. If you use metallizing, the zinc coating is sprayed directly onto the metal. Droplets cool, solidify and build up on the substrate into a laminar structure, forming a coating. Both of these methods have pros and cons.
Pros and Cons
One of the biggest benefits of using hot-dip galvanizing as opposed to metallizing is the ability to apply uniform protection, both on the inside and outside of the steel. The complete submersion process of hot-dip galvanizing allows for corrosion protection both inside and outside of the metal structure. With metallizing, for small diameter tubes, only the outside of the structure can be reached and the application could potentially be uneven, especially in corners. Therefore, hot-dip galvanizing is often the best choice for tubular metals or steel with a complicated shape because of the uniform protection it provides. Also, hot-dip galvanizing has a higher adhesion strength and abrasion resistance thanks to the metallurgical bond it creates between the steel and zinc. However, the somewhat rough surface the process of metallizing creates allows it to have a higher unprocessed slip coefficient, and allows paint to stick better to the surface of unprepared surfaces. Also, in situations where the steel material is large and thin, metallizing is a ‘cold’ process and can prevent deflection. A common guideline is to hot-dip galvanize material that does not have deflection concerns and fits into a kettle, and metallize those that don’t.
Another notable difference between the two is the location the process takes place. The hot-dip galvanizing process must take place inside a galvanizing plant, as the metal needs to be dipped in a specialized kettle, drained, and vented. With metallizing, the zinc coating can be applied at any location, with no draining or venting needed. This is a good option if the zinc coating needs to be applied on-site (referred to as field metallizing), or if the piece of metal being is too large to fit into a kettle. However, hot-dip galvanizing in a plant tends to be more economical all around.
Contact Duncan Galvanizing
Duncan Galvanizing is the Northeast’s premier hot-dip galvanizer, offering hot-dip galvanizing, factory applied powder and paint coatings, blasting, specification assistance and more. Contact us today at 617-389-8440 or fill out a contact form on our website.



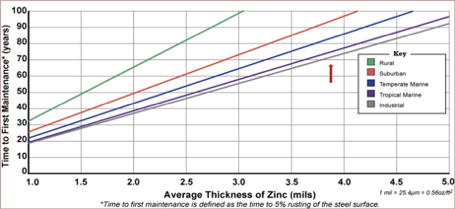



 The size of zinc baths varies from galvanizer to galvanizer and this restricts the size of steel that can be galvanized. When choosing a galvanizer, make sure that the size of the zinc bath is big enough to accommodate the size of your products such as large structural shapes.
The size of zinc baths varies from galvanizer to galvanizer and this restricts the size of steel that can be galvanized. When choosing a galvanizer, make sure that the size of the zinc bath is big enough to accommodate the size of your products such as large structural shapes.